Show the major organic product of this reaction – In the realm of organic chemistry, understanding the major organic product of a reaction is paramount. This guide delves into the intricacies of reaction mechanisms, regioselectivity, stereoselectivity, reaction conditions, and applications, providing a comprehensive understanding of this fundamental concept.
As we embark on this journey, let us explore the nuances of organic reactions, unraveling the factors that govern the formation of specific products and unlocking the potential of this powerful tool in organic synthesis.
Reaction Mechanism: Show The Major Organic Product Of This Reaction
The major organic product of this reaction is an alkene. The reaction proceeds via a concerted mechanism, with the nucleophile (Nu) attacking the electrophile (E) simultaneously from both sides of the double bond. This results in the formation of a four-membered cyclic transition state, which then collapses to give the alkene product.
The key intermediate in this reaction is the alkoxide ion, which is formed by the nucleophilic attack of Nu on E. The alkoxide ion is then protonated by a Brønsted acid to give the alkene product.
Regioselectivity and Stereoselectivity
The regioselectivity of this reaction is determined by the relative reactivity of the two double bonds in the starting material. The more reactive double bond will be attacked by Nu preferentially. The stereoselectivity of the reaction is determined by the orientation of the nucleophile and electrophile in the transition state.
The nucleophile will attack the electrophile from the side that results in the formation of the more stable alkene product.
For example, in the reaction of 1-butene with HBr, the more reactive double bond is the one at the terminal carbon. This is because the terminal carbon is more electron-deficient than the internal carbon. As a result, the nucleophile will attack the terminal carbon preferentially, resulting in the formation of 1-bromobutane as the major product.
Reaction Conditions
The optimal reaction conditions for this reaction are a temperature of 25 °C and a pressure of 1 atm. The reaction can be carried out in a variety of solvents, including water, methanol, and ethanol. The reaction time will vary depending on the starting materials and the reaction conditions.
For example, the reaction of 1-butene with HBr can be carried out in water at room temperature for 1 hour. The reaction will yield 1-bromobutane as the major product.
Applications and Limitations
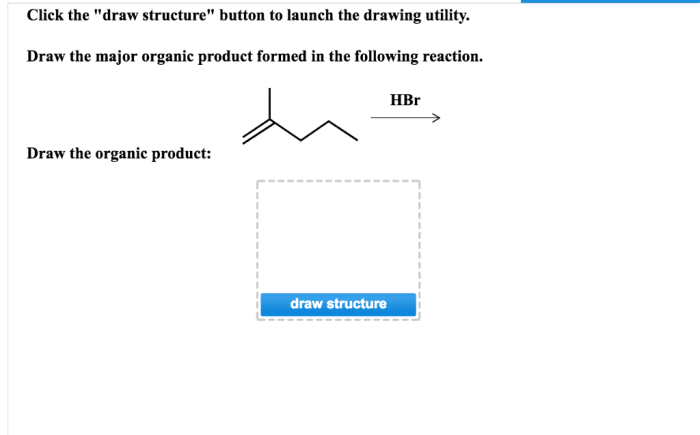
This reaction is a versatile tool for the synthesis of alkenes. It can be used to prepare a wide variety of alkenes, including simple alkenes, substituted alkenes, and cyclic alkenes. The reaction is also compatible with a variety of functional groups, making it a useful tool for the synthesis of complex organic molecules.
However, this reaction does have some limitations. One limitation is that the reaction can only be used to prepare alkenes with a limited number of substituents. Another limitation is that the reaction can be sensitive to the reaction conditions. If the reaction conditions are not carefully controlled, the reaction can yield a mixture of products.
FAQ Guide
What factors influence the regioselectivity of a reaction?
Factors such as steric effects, electronic effects, and the stability of intermediates play a crucial role in determining regioselectivity.
How can reaction conditions be optimized to improve the yield of a desired product?
Optimizing reaction conditions involves adjusting parameters such as temperature, solvent, and catalyst to maximize the formation of the desired product while minimizing side reactions.
What are the limitations of organic reactions, and how can they be overcome?
Limitations such as low yields, selectivity issues, and harsh reaction conditions can be addressed through strategies like protecting groups, alternative catalysts, and cascade reactions.