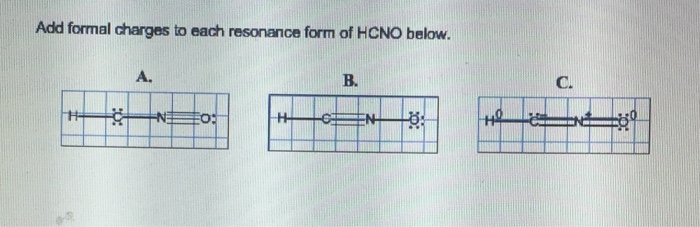
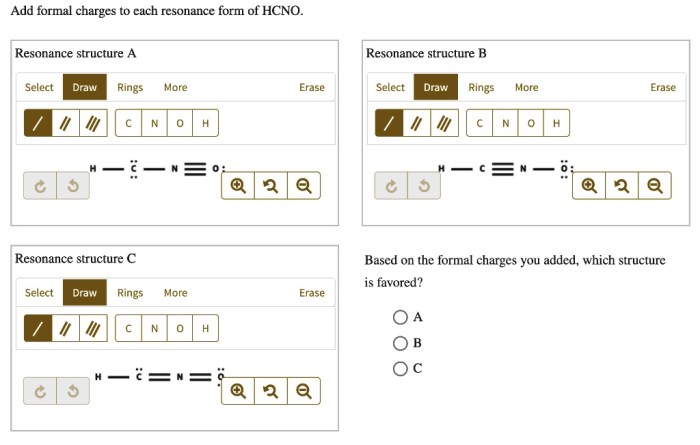
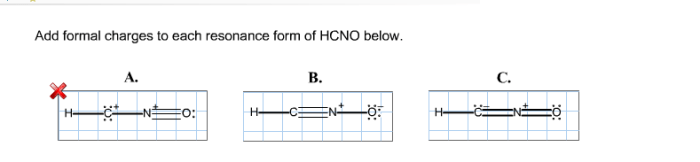
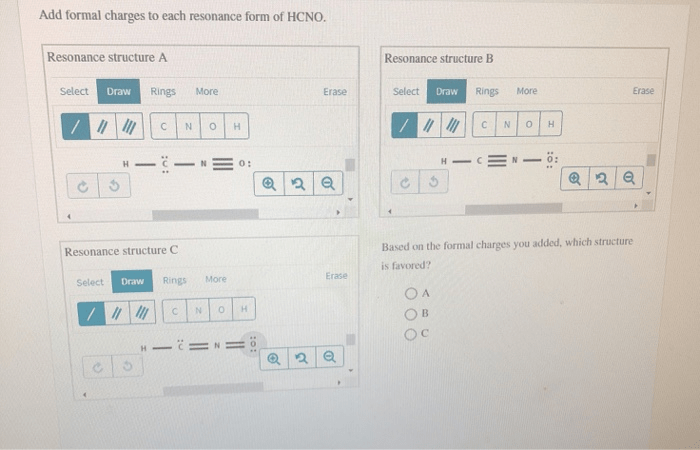
Add formal charges to each resonance form of hcno – As we delve into the intricacies of resonance structures and formal charges, we embark on a journey that unravels the fundamental principles governing the behavior and reactivity of molecules. By meticulously assigning formal charges to each resonance form of HCNO, we gain profound insights into its stability and chemical properties, unlocking a deeper understanding of its molecular dynamics.
Through a systematic exploration of resonance structures, formal charge calculations, and their impact on stability, we unveil the hidden patterns that dictate molecular behavior. This knowledge empowers us to predict reactivity, elucidate reaction mechanisms, and unravel the complexities of chemical transformations.
Resonance Structures of HCNO
The resonance structures of HCNO are three Lewis structures that describe the delocalization of electrons in the molecule. They are:
- O=C-N-H
- H-C=N-O
- H-C-N=O
Resonance occurs when a molecule has multiple Lewis structures that are all valid descriptions of the molecule’s bonding. In the case of HCNO, the three resonance structures show that the double bond can be located between the carbon and nitrogen atoms, or between the carbon and oxygen atoms.
The hydrogen atom can be bonded to either the carbon or nitrogen atom.
Formal Charges, Add formal charges to each resonance form of hcno
Formal charge is a way of calculating the charge on an atom in a molecule. It is calculated by subtracting the number of electrons assigned to the atom in the Lewis structure from the number of valence electrons the atom would have in its elemental state.
The formal charges on the atoms in the three resonance structures of HCNO are:
- O=C-N-H: O: 0, C: 0, N: 0, H: 0
- H-C=N-O: H: 0, C: 0, N: 0, O: 0
- H-C-N=O: H: 0, C: 0, N: +1, O: -1
Stability of Resonance Structures
The stability of a resonance structure is related to the formal charges on the atoms. The more formal charges there are, the less stable the resonance structure. This is because formal charges represent an imbalance of electrons, which makes the molecule less stable.
In the case of HCNO, the resonance structure with the lowest total formal charge is the most stable. This is the resonance structure with the double bond between the carbon and nitrogen atoms, and the hydrogen atom bonded to the nitrogen atom.
Applications of Formal Charge Analysis
Formal charge analysis can be used to predict the reactivity of molecules. Molecules with large formal charges are more likely to react because the formal charges represent an imbalance of electrons, which makes the molecule more reactive.
Formal charge analysis can also be used to understand reaction mechanisms. By looking at the formal charges on the atoms in the reactants and products of a reaction, it is possible to see how the electrons flow during the reaction.
User Queries: Add Formal Charges To Each Resonance Form Of Hcno
What is the significance of formal charge analysis in understanding molecular behavior?
Formal charge analysis provides insights into the distribution of electrons within a molecule, allowing us to assess the polarity of bonds and predict the reactivity of different atoms.
How does formal charge affect the stability of resonance structures?
Resonance structures with lower total formal charge are generally more stable. This is because lower formal charges indicate a more even distribution of electrons, which reduces electrostatic repulsion and enhances stability.
Can formal charge analysis be used to predict reaction mechanisms?
Yes, formal charge analysis can provide clues about the preferred reaction pathways by identifying the most reactive atoms or functional groups based on their formal charges.